Destination Specific Regulated Labels – Powered by Barcodes
Destination labeling is the process by which appropriate labels that contain necessary content are printed after the manufacturing and packaging steps, often during customer order fulfillment. Until recently, destination or deferred printing was a luxury. The destination labeling approach was largely the domain of larger companies that had the resources to implement the systems needed to make the process practical. But now with markedly increased regulatory and trade requirements around the world, destination labeling is becoming a mainstay. The good news is that developments in technology make destination labeling easier to implement. The FDA’s UDI (Unique Device Identification) barcode, and the powerful information it unlocks within enterprise systems, will be a key factor in implementing a robust and intelligent destination labeling system.
Destination Labeling is also known by a host of other names including, Labeling Localization, Just-In-Time Labeling, Print-on-Demand, and Country or Regionally Specific Labeling. Regardless of the name that is used, it is a response to label real estate shrinking while space requirements for language translations, regulatory and economic content, and symbologies on the label is expanding. This makes it very difficult to fit all the content within the limited space available on the majority of product packages.
The recently minted and demanding regulatory requirements of the European Union known as MDR (Medical Device Regulation) and IVDR (In Vitro Device Regulation), essentially force destination labeling capabilities. But what is destination labeling all about at its very essence? In short, it is the production of regionally specific labels to meet localized regulatory requirements. There are many regulatory challenges for shipping life sciences (medical) products that destination labeling can solve. They include a need to:
- Fit close to two dozen language translations for multiple lines of text on smaller boxes
- Print specific registration numbers when a product is sold in China or Russia
- Print local contact information for importers and other economic operators
- Handle a growing list of diverging local regulations and commercial requirements
- Provide small durable implant cards in the language of the recipient
- Print the MSRP (Manufacturer’s Suggested Retail Price) in Rupees, and other information locally when a product is sold in India
As you can see by this list, country or regionally specific requirements are the main driver for destination labeling. Some countries even require that product be shipped to bonded warehouses in-country, and these products must be labeled before passing customs. As countries, regions, and regulatory bodies continue to improve patient safety and increase organized control over the distribution of medical products, localized labeling becomes more important.

Products are shipping to places in the world that require content in the local language. Some life sciences companies have product labels that are as long as 17” or more. They do this to fit in multiple lines of text containing dozens of languages. Not every product has packaging that will support this.
How is destination labeling accomplished?
The objective is to perform labeling operations only once for as many products as possible. Only the exceptions should be touched a second or more times. Assuming that one SKU is used to identify a product in all international markets, it has been reported that about 85% of products can reach final destination utilizing a CLAD (Core Languages All Destinations) universal label. This label is applied at the production facility. The manufacturer chooses a set of languages for destinations that it commonly ships most of its products to and includes these languages on the CLAD label; often these are- English, French, Italian, German, and Spanish. After satisfying CLAD requests, there is a need to identify destinations that are not covered by the CLAD. This is done at order fulfilment time, if needed, printing destination specific labels and IFUs (instructions for use).
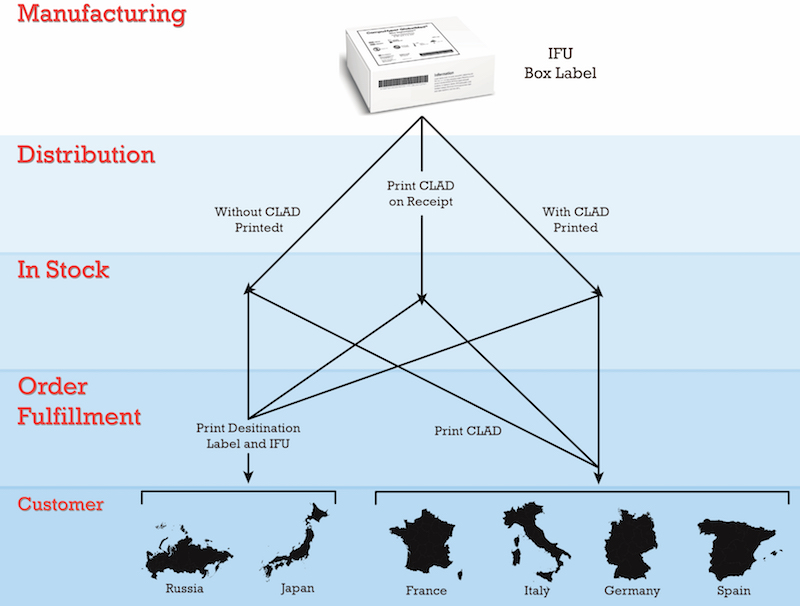
Systems are needed at order fulfillment locations to link product being picked for a customer order with labeling content management systems. Scanning a barcode on a box or bin location during order fulfillment causes a destination labeling system to match it against the pick list detail. An appropriately constructed system will have the intelligence to say, “You are going to Russia. I need to print the Russian label at the appropriate label printer and print the Russian IFU at the appropriate document printer.” On occasion, a destination requires multiple languages on the label and IFU. What is needed to enable this intelligence is a data management system to house the product information for each SKU, language requirements for each destination, supporting PDFs of IFUs, and other important pieces of information. Links between items, their potential destinations and associated documentation must also be established and become electronic business rules. Linkages then drive the fulfillment process in an automated way once the UDI barcode is scanned from which the SKU, the LOT/Serial number, and potentially the expiration date is derived.
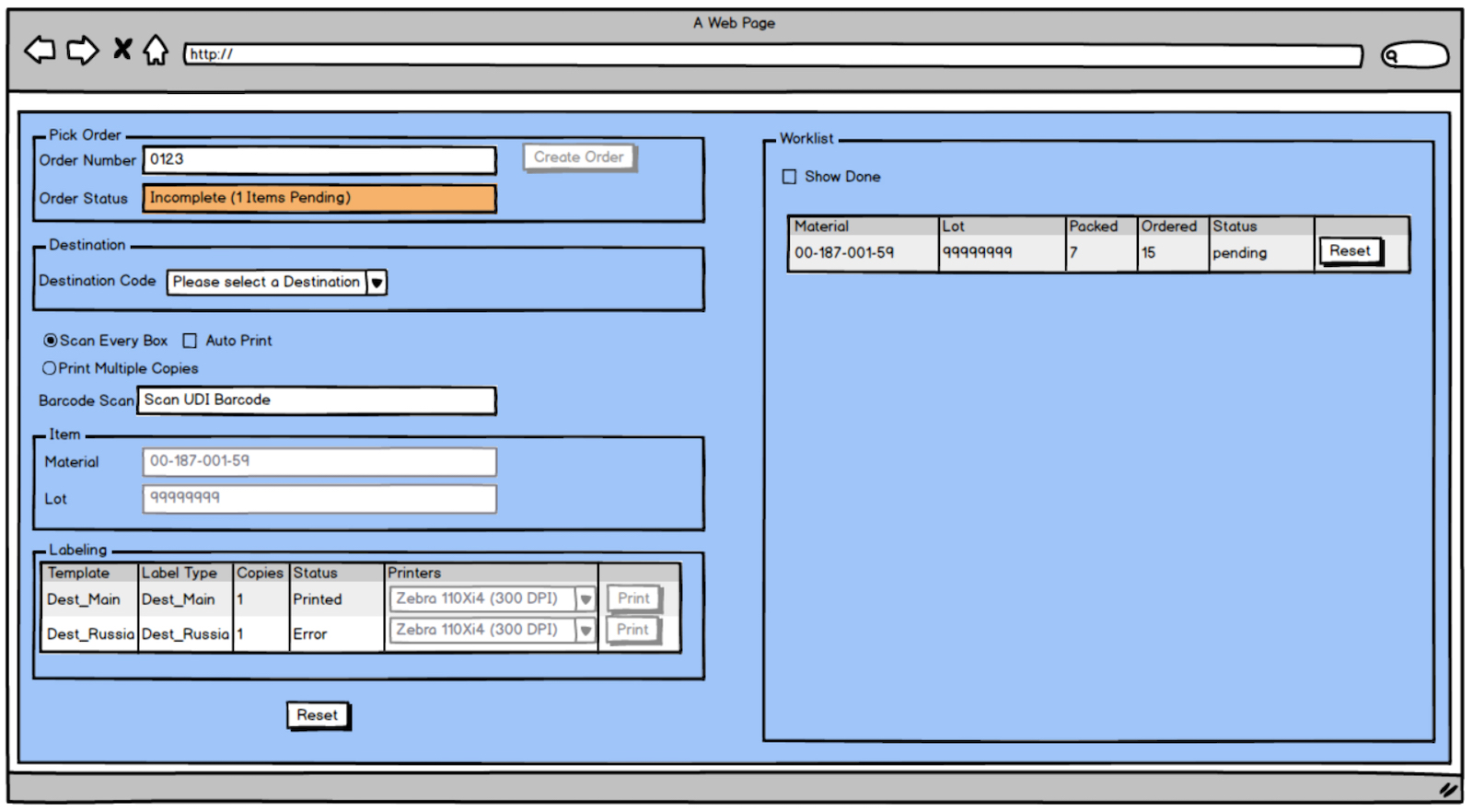
Destination labeling systems can convert linkages (associations) made in a Master Data Management system into automated business rules. Barcode scanning drives integration to pick lists to complete the order fulfillment process for delivery anywhere in the world. Based on configuration, the system decides whether additional labels and documents need to be printed.
The format of the printed label(s) is specific to SKU and destination.
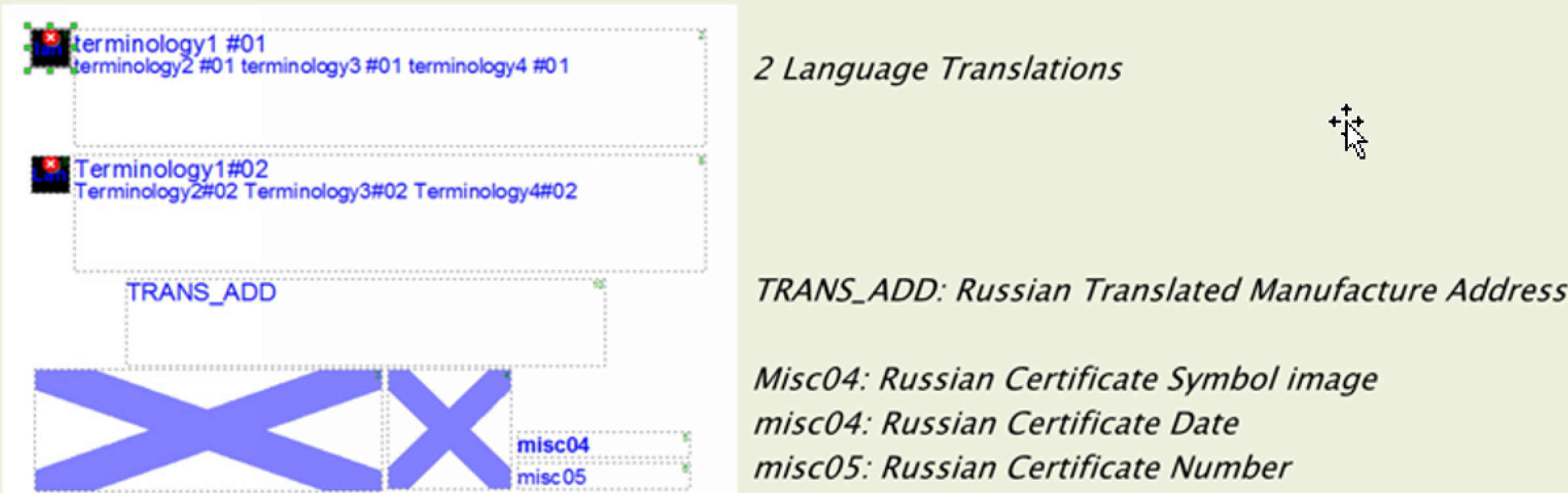
Local language label, the region, and the product number. Labeling templates contain fields that are filled from information in a database. This includes language translations and symbols.
Exploiting the power of barcode triggered linkages requires the capability to make one-to-many and many-to-many associations between items, templates, documents, regions, countries, or CLAD. Associated regulatory images and language translations must be carried along with all of this. For example, EU MDR/IVDR will require translations into any of 24 languages if shipping to EU countries. A system should be able to associate these language translations with items and templates that have been appropriately designed to house them. Significant process analysis is required when setting up a system like this. However, the outcome can be tremendous. An appropriately designed system can:
- Upload and store IFUs (Instructions for Use) and other documents
- Link IFUs to item versions and destinations
- Manage label templates by item/destination
- Maintain destination language translations in a dictionary
- Deliver a browser-based page to read order fulfillment pick-lists and print documents based on user input/scan
Summary
With expanded globalization and diligent work by regulatory thought leaders in the life science industry, everyone was hoping that there would be a convergence and harmonization of regulatory requirements concerning labeling. However, indications are to the contrary. Stakeholders, responding to their national and regional requirements, are clearly diverging from the concept of a universal label. Destination labeling systems can alleviate the current and growing challenges of international order fulfillment where inventory turns can be maximized with no loss in traceability or accountability. Regulatory bodies will keep adding complexity, and we, as barcode and labeling practitioners, must have systems that can handle these new challenges through an agile, powerful, and configurable system.
By Ardi Batmanghelidj, President and CEO, Innovatum, Inc

Over the past 30+ years, Ardi Batmanghelidj has held several leadership positions in the software industry. Since 1994 the concentration of Ardi’s work has been in the Life Science vertical with emphasis on regulatory compliance. His extensive hands on experience implementing and integrating ERP, MES, and labeling systems benefits Innovatum customers both in the capabilities of Innovatum software and during the implementation phase of any project. Ardi is a published author and sought-after speaker at industry forums, providing expert guidance to the FDA and manufacturers in the area of master data management, labeling and barcoding. Most recently as the Chairman of the AIM North America Healthcare committee, and as one of the authors of the AIM white paper on UDI, Ardi has been immersed in gauging the impact of serialization and Global UDI initiatives and has been assisting the industry toward compliance.
Ardi Batmanghelidj holds a Bachelor’s degree in Economics and Political Science from the University of Massachusetts. He has additional IT certifications from the Computer Processing Institute, Boston University, and the Worcester Polytechnic Institute
--
The Bar Code News is now in its 9th year and is pleased to bring you great exclusive content. We rely on sponsors to keep this website running, so please let vendors know that you've seen them here. Thank you. Is your bar code related business listed in our free directory? Over 500,000 visitors a year come to Barcode.com. It pays to be here.
Find us on social media too:
https://facebook.com/theBarCodeNews
https://twitter.com/theBarCodeNews
