2D Barcodes for Vaccines

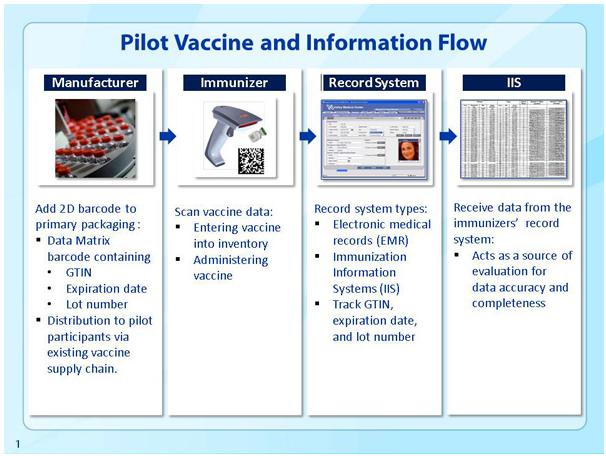
In a completed pilot project, which ran from September 2011 to May 2013, the Centers for Disease Control (CDC) tested the implementation of 2D barcodes on vaccine products. The project involved vaccines from two different manufacturers and required them to apply 2D barcodes onto specific vaccine products; the barcoded vaccines were then distributed to a preselected network of pilot project immunization providers. The barcoded vaccines were shipped through the existing supply chain to 220 different immunizers across 10 different CDC grantee locations.
The specialized 2D barcode placed on each vaccine product contained the following data: a product identifier, lot number, and expiration date. Providers used barcode scanners to read the 2D barcodes and automatically imported the data from those barcodes into the patients’ electronic medical records (EMRs). In addition to the patient-level information record, the CDC maintains a larger database of information about immunizations in the greater population. These systems, immunization information systems, are “confidential, population-based, computerized databases that record all immunization doses administered by participating providers to persons residing within a given geopolitical area.”
Clearly, the data obtained from these 2D barcodes is vitally important not only to the individual patient and her medical records but also to the public health community at large. When tracking diseases and epidemics, immunization information is a hugely important resource. According to the CDC, “The goal of this pilot implementation of 2D barcoded vaccines and scanners was to assess their impact on the completeness and accuracy of vaccine data in both the EHR [electronic health record] and the IIS [immunization information system].”
In light of questions connections to autism, as well as the need for full vendor and manufacturer accountability it is easy to understand why vaccines are so strictly regulated and data is so carefully recorded from manufacturer to patient. The National Childhood Vaccine Injury Act (NCVIA) requires that vaccine production information and lot numbers be documented. In the existing model, this information is usually handwritten or typed into an EMR system or the larger IIS system. The CDC reports that under this model, these data “are frequently missing or incorrect within IIS and Vaccine Adverse Event Reporting System (VAERS) reports.” The idea behind a standardized, easily implemented 2D barcoding system is to allow for efficient and accurate recording of these data and seamless integration into both the patient’s EMR and the larger IIS database.
A clear benefit of the 2D barcode over more traditional linear barcodes is that the 2D barcode can encode all the required information in a smaller footprint. These tiny, square-shaped 2D barcodes can store not only a vaccine’s product information but also its lot number and expiration date. A linear barcode can currently only support vaccine product information. An NIH study forecast savings of several hundred million dollars over the next ten years as a result of using 2d barcodes, as well as improved immunization data quality.
In addition to the pilot program implementing 2D barcodes on vaccines themselves, the CDC has also implemented a barcoding system for its Vaccine Information Statement (VIS) program. In keeping with the more informed patient public (and federal law), Vaccine Information Statements (VISs) are “information sheets produced by the CDC that explain both the benefits and risks of a vaccine to vaccine recipients… federal law requires that healthcare staff provide a VIS to a patient, parent, or legal representative before each dose of certain vaccinations.” The CDC has already implemented 2D barcodes on all its VIS sheets as part of its “modernization initiative.”
The 2D barcodes on VISs require a 2D-compatible barcode scanner as well as software to read the data in the 2D VIS barcodes. For medical offices already operating with some form of electronic medical records (EMRs), it is unlikely that major upgrades or expenses will be required in order to get up and running scanning the 2D VIS barcode information. For providers using paper record systems, the 2D barcodes are not required, and the same information can still be manually entered into patient records. The CDC is quick to note that the 2D barcodes on VIS sheets are optional and that providers not prepared to implement the technology need not fear the consequences of not having the required equipment. However, possible benefits of using the 2D VIS barcodes include increased efficiency and accuracy: “Scanning the VIS barcode may reduce the time needed to record the VIS information and, more importantly, also reduce the chance of errors in transcribing this information. Additionally, in the future electronic medical records (EMR) vendors may support reading the VIS barcode into the EMR system and validate it against the vaccine selected for, or administered to the patient.”
An important point to remember about the 2D barcode vaccine pilot program and the fuller VIS 2D barcode system is that the two systems are not related, and data from one does not relate to the other. The CDC makes it clear that there is “no relationship” between the VIS barcodes and 2D barcodes on vaccine products like vials and syringes. The different information contained in the two barcodes means they are unrelated and incompatible.
The healthcare system in our country is rapidly changing, and just as providers are getting used to one system, the Affordable Care Act ( “Obamacare”) is poised to come online in the next few months. With all these shifting standards, there is a definite need for standardized immunization record systems that are efficient, accurate, and compatible with new EMR technology. The 2D barcodes implemented in both the CDC pilot program for vaccine vials and syringes and those now appearing on all VIS sheets mean quick access to accurate, up-to-date information without the risk of human recording error. When human lives are at risk, these barcodes can make a big difference in at both the patient and public health levels.
Written by Kathryn Cunningham
Sources:
CDC.gov - Centers for Disease Control and Prevention
National Institutes of Health - National Center for Biotechnology Information